Research Areas
Engineering Proteins to Modulate the Immune System
Immune system engagement is a powerful approach that is being exploited to treat cancer and other diseases. We pursue understudied immune targets with direct translational impact. As examples, we engineer novel proteins and antibodies for tumor and stromal targeted immuno-modulation and collaborate with experts to use engineering strategies to develop enhanced cell therapies. Our work has contributed to therapeutic candidates that are advancing towards clinical testing and includes elucidation of structural features and binding/functional properties.
Engineering Natural Receptor-Ligand Interactions to Create Therapeutics
The biological importance and druggable properties of receptors and their cognate ligands have designated them as especially useful clinical targets. This significance continues to expand as molecular insights underlying disease are uncovered. Our lab has focused on developing novel tailor-made protein therapeutics against ligand and receptor targets implicated in cancer and regenerative medicine. At our core, we are committed to basic science pursuits, developing these engineered proteins as tools to correlate sequence-structure-function relationships and provide mechanistic insight into ligand/receptor interactions. Importantly, we also have a passion for bench-to-bedside research that has the potential to greatly impact patients. Towards this end, a number of the engineered proteins developed in our lab have moved forward towards clinical development for oncology or regenerative medicine applications.
Cancer
Antibodies are therapeutic workhorses of the pharmaceutical industry, and have been generated against ligands and receptors to inhibit tumor cell signaling, growth, and metastasis. Instead, we engineer the ligands and receptors themselves as therapeutics to overcome inherent limitations of antibodies and offer novel therapeutic approaches for cancer. As examples, we have used rational and combinatorial methods to engineer natural ligands, including VEGF, HGF, CLCF1, HAI-1, and PDGF as high affinity, potent inhibitors of cancer cell function. In addition, we have engineered designer versions of soluble receptors (aka receptor ‘decoys’) to bind to and sequester ligands that would otherwise drive cell signaling and cancer progression. These engineered receptor decoys overcome inherent high affinity and complexity of natural ligand-receptor interactions. Examples include an engineered Axl receptor decoy that binds with ultra-high affinity (fM) to Gas6 and inhibits metastatic spread of aggressive cancers (currently in Phase Ib/II trials for ovarian and clear cell renal cancer), and engineered CNTFR and LIFR decoys that bind with high affinity and inhibit activity of CLCF1 and LIF ligands, respectively, for treatment of lung and pancreatic cancers.
*VEGF= vascular endothelial growth factor; HGF= hepatocyte growth factor; CLCF1= cardiotrophin like cytokine factor 1; HAI-1= hepatocyte growth factor activator inhibitor type-1; PDGF= platelet-derived growth factor; Axl= Axl receptor tyrosine kinase; Gas6= growth arrest specific 6; CNTFR= ciliary neurotrophin factor receptor; LIFR= leukemia inhibitory factor receptor; LIF= leukemia inhibitory factor.
Regenerative Medicine
We also engineer natural ligands to serve as potent stimulators of cell activation for regenerative properties in wound healing, cardiac tissue regeneration, and ophthalmic applications. Examples include engineered epidermal growth factor (EGF) mutants with enhanced activity, which are currently under commercial development for wound healing and cosmetic applications; a stable engineered protein fragment of HGF that has shown promise in repairing tissues following myocardial infarction (i.e. heart attack) in rodent and sheep models; an engineered CLCF1 ‘superagonist’ that stimulates enhanced neural cell regeneration, and engineered glucagon-like peptide 1 (GLP-1) agonists for insulin modulation in diabetes. We collaborate with a number of leading researcher groups who develop novel biomaterials for optimal delivery of these engineered growth factors and cytokines to places they are needed in the body.
Biophysical Characterization of Engineered and Native Proteins
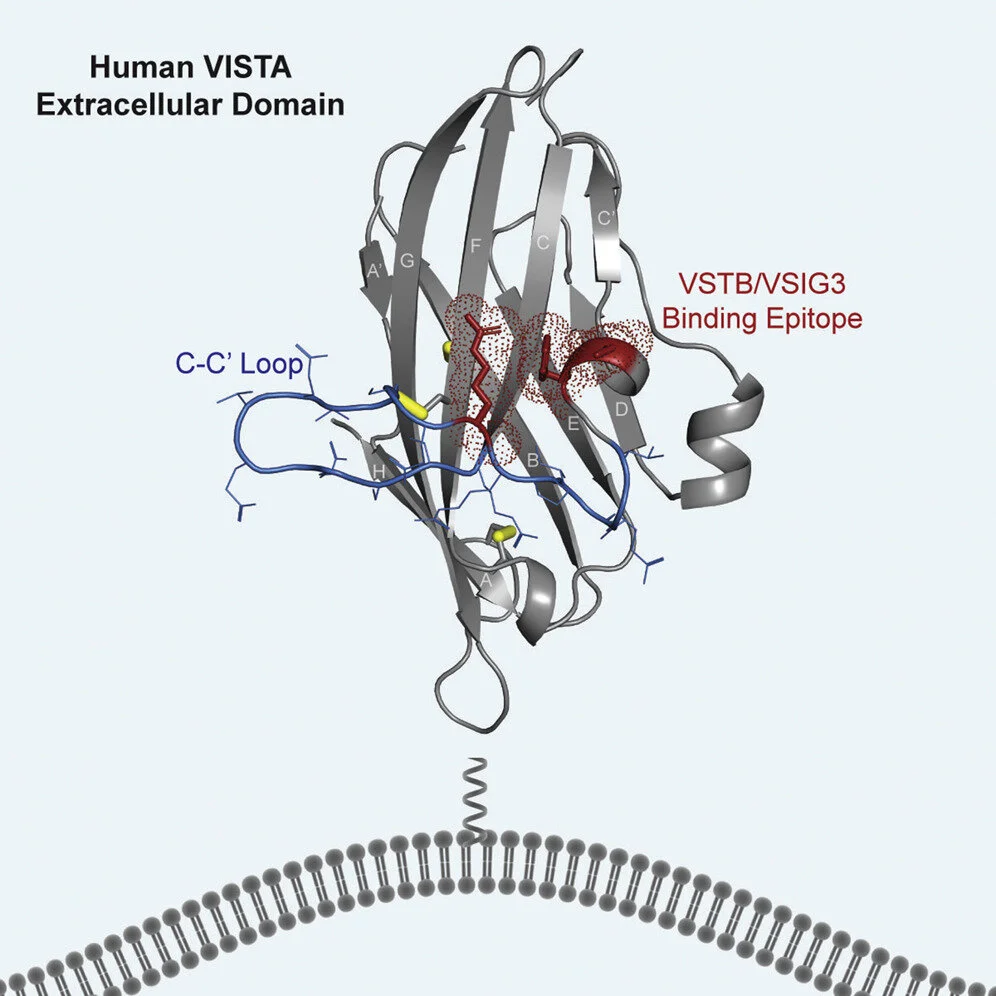
We are interested in understanding the molecular structure and interactions of the proteins that we engineer. We collaborate with scientists at the Stanford Linear Accelerator Center (SLAC) to discover new protein structures through x-ray crystallography and cryo-EM. We also use epitope mapping to determine important binding residues for our antibodies. We were the first to publish the crystal structure of VISTA, an important and understudied immune checkpoint protein that inhibits the T cell response against cancer.
Applications of Integrin Targeting Cystine Knot Peptides (knottins)
Our research group has a strong interest in developing peptide-based alternatives to monoclonal antibodies for tumor-targeting applications. Towards this goal, we engineered cystine knot (knottin) peptides for high affinity molecular recognition against tumor-associated receptors. The knottin family of peptides contains a disulfide-bonded core that confers outstanding proteolytic resistance and thermal stability. We have engineered a knottin, 2.5F, that binds to multiple integrin pairs upregulated on cancer cells. The peptide benefits from increased tumor permeability and rapid clearance from non-tumor tissues due to its small (3 kDa) size. 2.5F has been used in numerous applications for imaging and drug delivery. It has also been studied as a combination therapy through blockade of integrins or through Fc-dependent immune activation through the creation of a 2.5F Fc fusion.