Engineered CD47 protects T cells for enhanced antitumor immunity
in Nature on May 15, 2024
by Sean A. Yamada-Hunter,Johanna Theruvath, Brianna J. McIntosh, Katherine A. Freitas, Frank Lin, Molly T. Radosevich, Amaury Leruste, Shaurya Dhingra , Naiara Martinez-Velez, Peng Xu, Jing Huang, Alberto Delaidelli, Moksha H. Desai, Zinaida Good, Roel Polak, Audre May, Louai Labanieh, Jeremy Bjelajac, Tara Murty, Zach Ehlinger , Christopher W. Mount, Yiyun Chen, Sabine Heitzeneder, Kristopher D. Marjon, Allison Banuelos, Omair Khan, Savannah L. Wasserman, Jay Y. Spiegel, Sebastian Fernandez-Pol, Calvin J. Kuo , Poul H. Sorensen, Michelle Monje, Robbie G. Majzner, Irving L. Weissman, Bita Sahaf, Elena Sotillo, Jennifer R. Cochran, and Crystal L. Mackall
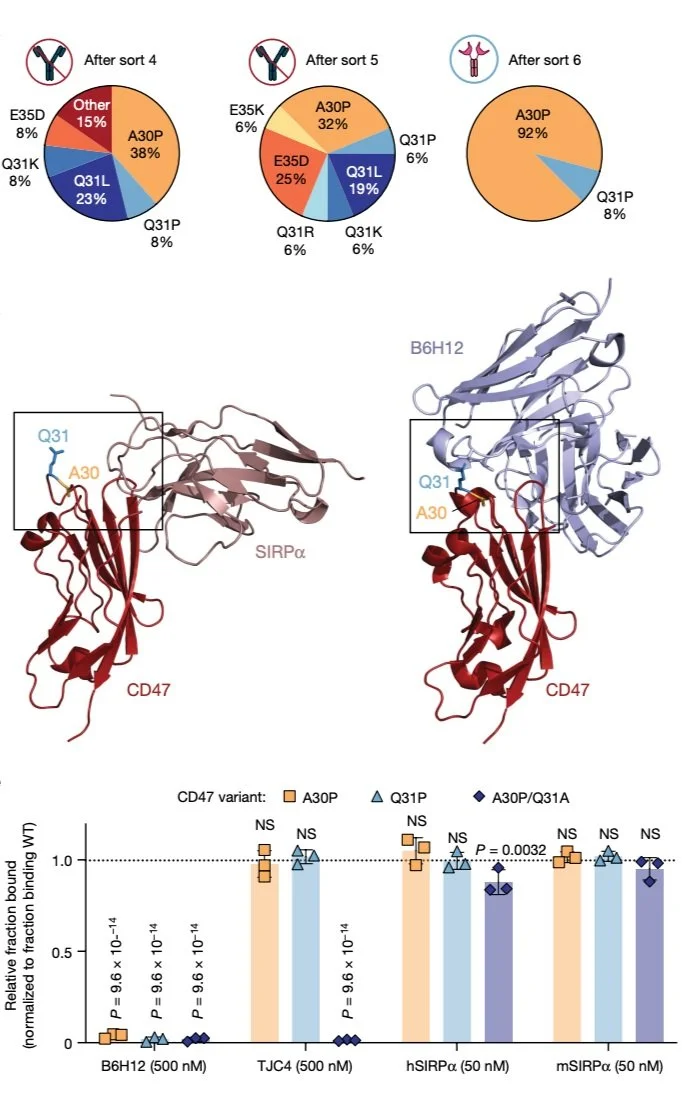
Adoptively transferred T cells and agents designed to block the CD47–SIRPα axis are promising cancer therapeutics that activate distinct arms of the immune system. Here we administered anti-CD47 antibodies in combination with adoptively transferred T cells with the goal of enhancing antitumor efficacy but observed abrogated therapeutic benefit due to rapid macrophage-mediated clearance of T cells expressing chimeric antigen receptors (CARs) or engineered T cell receptors. Anti-CD47-antibody-mediated CAR T cell clearance was potent and rapid enough to serve as an effective safety switch. To overcome this challenge, we engineered the CD47 variant CD47(Q31P) (47E), which engages SIRPα and provides a ‘don’t eat me’ signal that is not blocked by anti-CD47 antibodies. TCR or CAR T cells expressing 47E are resistant to clearance by macrophages after treatment with anti-CD47 antibodies, and mediate substantial, sustained macrophage recruitment to the tumor microenvironment. Although many of the recruited macrophages manifested an M2-like profile, the combined therapy synergistically enhanced antitumor efficacy. Our study identifies macrophages as major regulators of T cell persistence and illustrates the fundamental challenge of combining T-cell-directed therapeutics with those designed to activate macrophages. It delivers a therapeutic approach that is capable of simultaneously harnessing the antitumor effects of T cells and macrophages, offering enhanced potency against solid tumors.
An Engineered NKp46 Antibody for Construction of Multi-Specific NK Cell Engagers
in Protein Eng Des Sel, 2024
by Robert B Lee, Sainiteesh Maddineni, Madeleine Landry, Celeste Diaz, Aanya Tashfeen, Sean A. Yamada-Hunter, Crystal L. Mackall, Corinne Beinat, John B. Sunwoo, and Jennifer R. Cochran
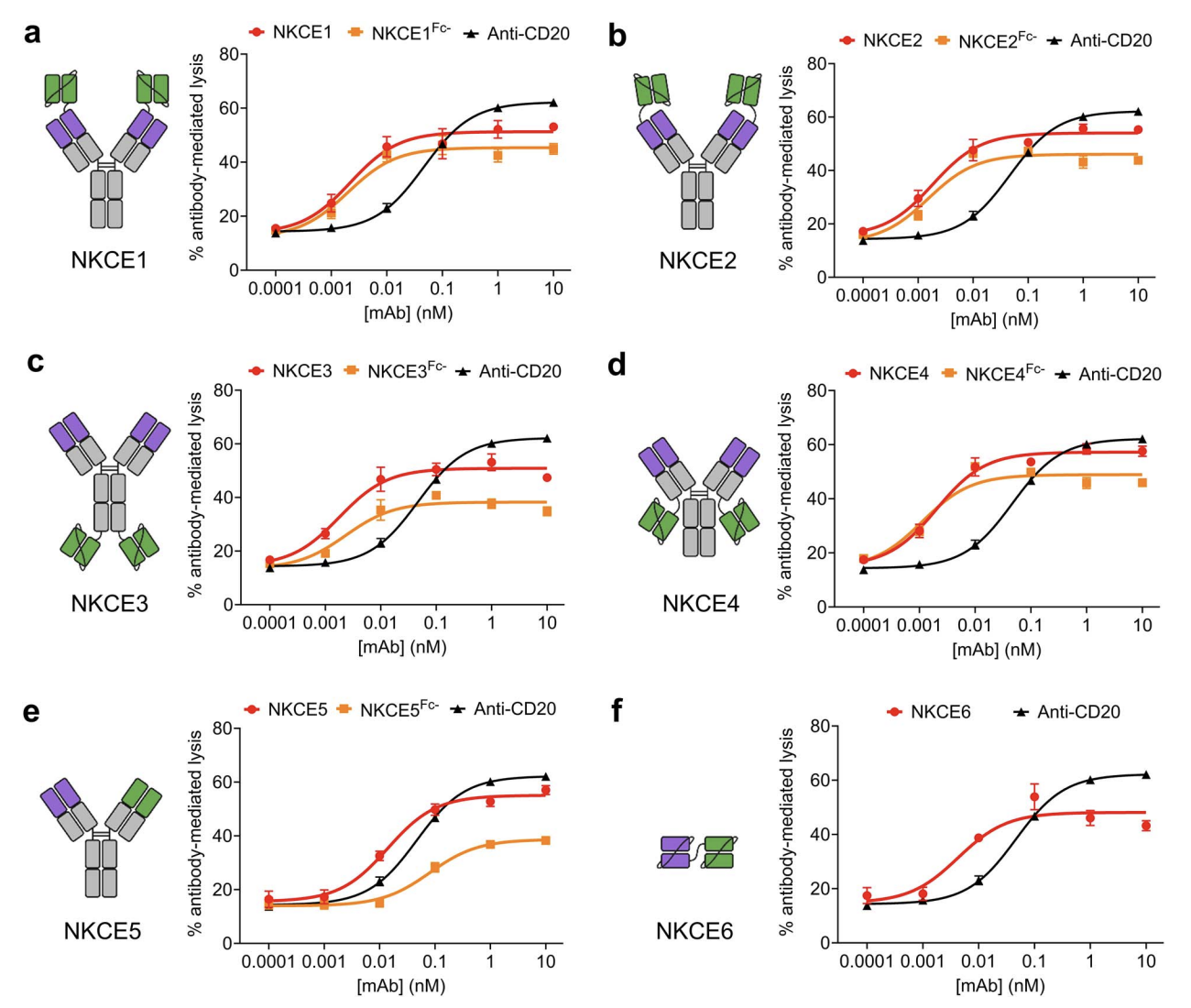
Recent developments in cancer immunotherapy have highlighted the potential of harnessing natural killer (NK) cells in the treatment of neoplastic malignancies. Of these, bispecific antibodies, and NK cell engager (NKCE) protein therapeutics in particular, have been of interest. Here, we used phage display and yeast surface display to engineer RLN131, a unique cross-reactive antibody that binds to human, mouse, and cynomolgus NKp46, an activating receptor found on NK cells. RLN131 induced proliferation and activation of primary NK cells, and was used to create bispecific NKCE constructs of varying configurations and valency. All NKCEs were able to promote greater NK cell cytotoxicity against tumor cells than an unmodified anti-CD20 monoclonal antibody, and activity was observed irrespective of whether the constructs contained a functional Fc domain. Competition binding and fine epitope mapping studies were used to demonstrate that RLN131 binds to a conserved epitope on NKp46, underlying its species cross-reactivity.
Structural insights reveal interplay between LAG-3 homodimerization, ligand binding, and function
in PNAS on March 14, 2024
by Jack L Silberstein, Jasper Du, Kun-Wei Chan, Jessica A Frank, Irimpan I Mathews, Yong Bin Kim, Jia You, Qiao Liu, Elliot A Philips, Phillip Liu, Eric Rao, Daniel Fernandez, Grayson E Rodriguez, Xiang-Peng Kong, Jun Wang, and Jennifer R Cochran
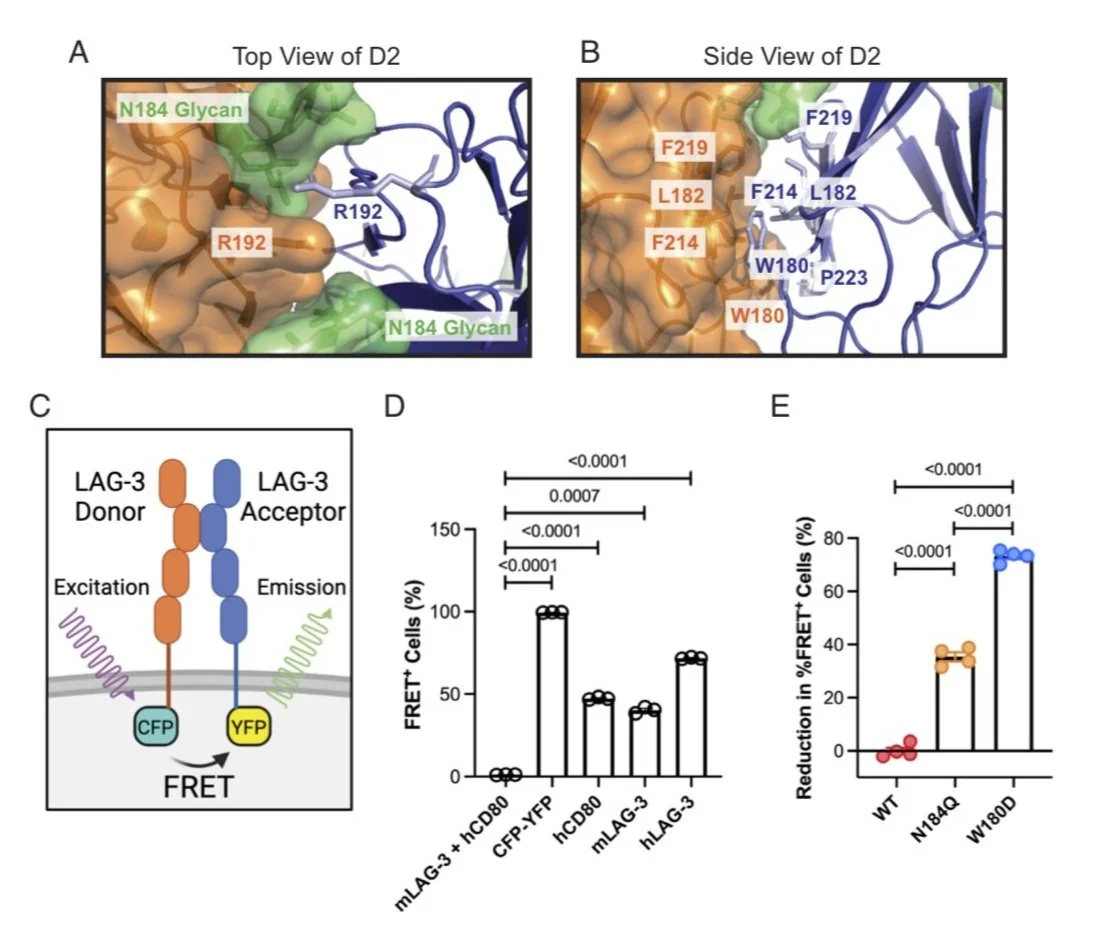
Lymphocyte activation gene-3 (LAG-3) is an inhibitory receptor expressed on activated T cells and an emerging immunotherapy target. Domain 1 (D1) of LAG-3, which has been purported to directly interact with major histocompatibility complex class II (MHCII) and fibrinogen-like protein 1 (FGL1), has been the major focus for the development of therapeutic antibodies that inhibit LAG-3 receptor-ligand interactions and restore T cell function. Here, we present a high-resolution structure of glycosylated mouse LAG-3 ectodomain, identifying that cis-homodimerization, mediated through a network of hydrophobic residues within domain 2 (D2), is critically required for LAG-3 function. Additionally, we found a previously unidentified key protein-glycan interaction in the dimer interface that affects the spatial orientation of the neighboring D1 domain. Mutation of LAG-3 D2 residues reduced dimer formation, dramatically abolished LAG-3 binding to both MHCII and FGL1 ligands, and consequentially inhibited the role of LAG-3 in suppressing T cell responses. Intriguingly, we showed that antibodies directed against D1, D2, and D3 domains are all capable of blocking LAG-3 dimer formation and MHCII and FGL-1 ligand binding, suggesting a potential allosteric model of LAG-3 function tightly regulated by dimerization. Furthermore, our work reveals unique epitopes, in addition to D1, that can be targeted for immunotherapy of cancer and other human diseases.
An engineered interleukin-11 decoy cytokine inhibits receptor signaling and proliferation in lung adenocarcinoma
in Bioengineering & Translational Medicine on July 18, 2023
by Brianna J. McIntosh, Griffin G. Hartmann, Sean A. Yamada-Hunter, Phillip Liu, Camille F. Williams, Julien Sage Jennifer R. Cochran
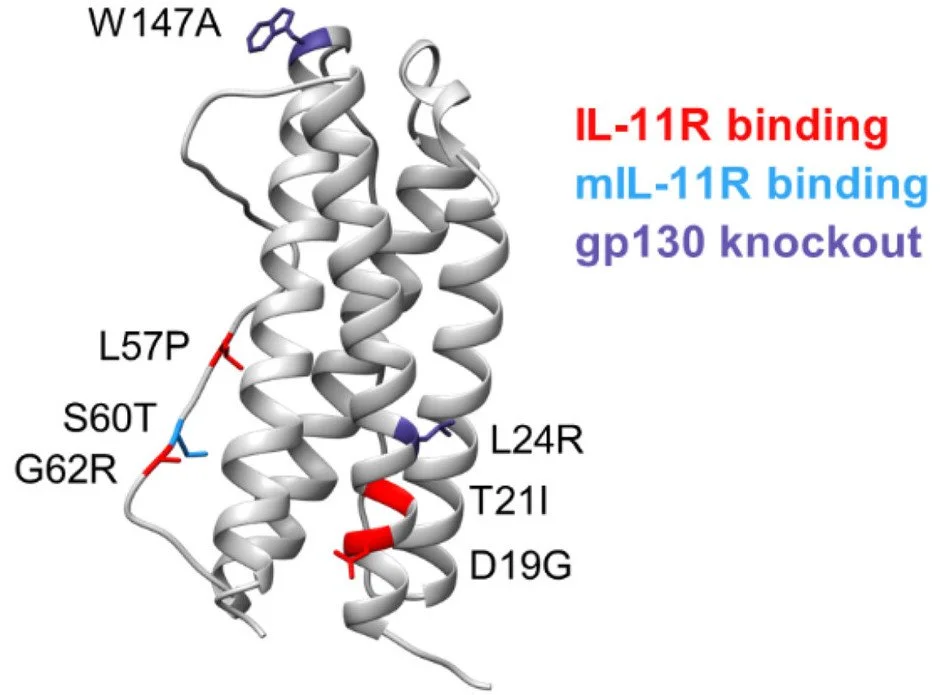
The cytokine interleukin (IL)-11 has been shown to play a role in promoting fibrosis and cancer, including lung adenocarcinoma, garnering interest as an attractive target for therapeutic intervention. We used combinatorial methods to engineer an IL-11 variant that binds with higher affinity to the IL-11 receptor and stimulates enhanced receptor-mediated cell signaling. Introduction of two additional point mutations ablates IL-11 ligand/receptor association with the gp130 coreceptor signaling complex, resulting in a high-affinity receptor antagonist. Unlike wild-type IL-11, this engineered variant potently blocks IL-11-mediated cell signaling and slows tumor growth in a mouse model of lung cancer. Our approach highlights a strategy where native ligands can be engineered and exploited to create potent receptor antagonists.